Customer: Accredo Health Solutions
Location: Memphis, TN
Project Summary
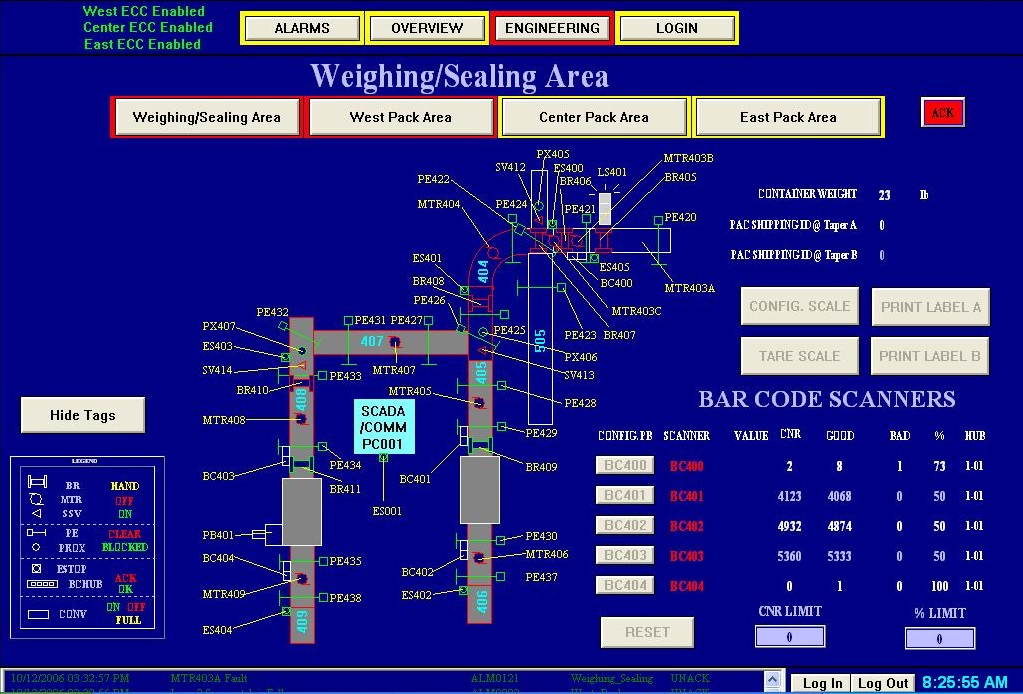
Accredo Health Solutions required a turnkey order consolidation, case packaging, and conveying system with case closure, labeling, and verification. Each case needed to receive a pre-printed serialized license plate along with a separate customer shipping label.
GCI Solution
GCI worked closely with the Accredo corporate engineering group to develop a materials handling solution designed with physical space constraints for both infeed components as well as out feed full cases. This system incorporated:
- Driven roller conveyor
- O-ring pop-up transfers
- Belt conveyor sections
- In-line check weighing
- APAL station(s) with Sato Thermal Transfer Engine
- Unattended barcode scanning
- Automated box taping
Five hundred feet of conveyor, along with two automatic case sealers, a checkweigher, and ten packing stations were fit into an area of 3600 square feet, with no overlapping lanes. GCI authored and executed a full quality plan including Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ) test protocol.

APAL Station with Box Sealer
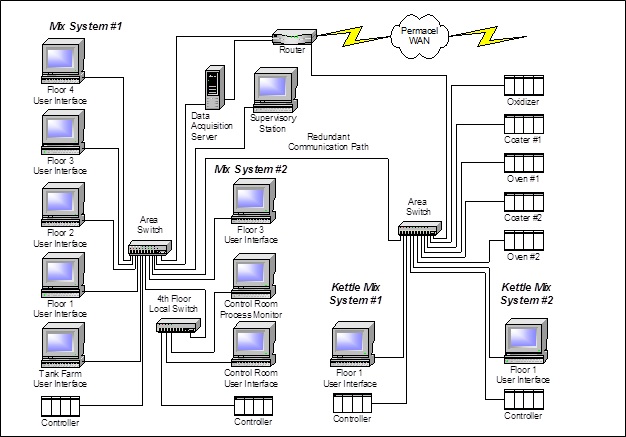
The System was outfitted with an Allen Bradley ControlLogix PLC for conveyor control and an Invensys Wonderware SCADA node for HMI, manual control, annunciation and recovery. The print engine, ERP interface and execution system for label printing and order weight verification were designed and implemented as background services on the same platform.
As a result of these improvements, shipping capacity was increased from 3000 units per day to 10000 units per day.